Electrolytic copper equipment process for blister copper refining
Electrolytic copper can be divided into two grades: high-purity electrolytic copper (Cu-CATH-1) and standard electrolytic copper (Cu-CATH-2). The purity of electrolytic copper is extremely high, and the copper content can reach 99%. Electrolytic copper is a copper foil formed by adsorbing copper ions on a substrate by electrolysis. Electrolytic copper can use oxygen-free copper, scrap copper, etc. as raw materials. How many steps are there in the electrolytic copper technology?
The existing waste copper recycling process has problems such as long process flow, low recovery rate, large investment, high energy consumption, low utilization level, and large environmental pollution. Therefore, research and development of waste copper direct electrolysis without smelting casting process Refining the clean production process has become an urgent task.
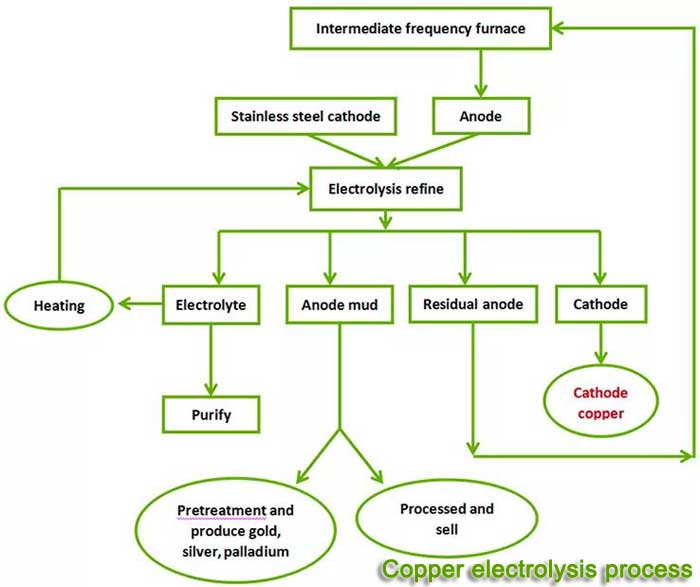
The clean production process of direct electrolytic refining of scrap copper is based on scrap copper as raw material:
1. Put the raw material into the anode frame as the anode, and use the permanent stainless steel cathode plate or pure copper sheet as the cathode.
2. Use acid copper sulfate solution as electrolyte for direct electrolytic refining of waste copper.
3. During the electrolysis process, the copper on the anode loses two electrons to form -2-valent copper ions into the solution, while the precious metals and some metals are insoluble and become anode slime and precipitate at the bottom of the electrolytic cell.
4. The -2valent copper ions in the solution are preferentially precipitated on the cathode
5. Other base metals with negative potential cannot be precipitated on the cathode and remain in the electrolyte to be removed when the electrolyte is regularly purified.
Therefore, electrolytic refining can obtain high-grade electrolytic copper and anode slime enriched with precious metals.
During the copper electrolysis process, the content of copper and impurities in the electrolyte gradually increases, the content of additives accumulates, and the content of sulfuric acid gradually decreases, so that the composition of the electrolyte deviates from the required condition control range. Therefore, it is necessary to periodically and quantitatively extract the electrolyte for purification treatment, and replace it with an equal amount of new liquid to adjust the composition of the electrolyte; at the same time, valuable metal impurities are also recovered to ensure the normal operation of electrolytic refining.
Electrolytic copper technology process principle:
Due to ionization, the components in the electrolyte react to form ions
When no power is applied, the above reactions are in dynamic equilibrium. However, in the case of direct current passing through the electrodes and the solution, various ions make directional movements and may react on the anode.
The precipitation of oxygen also has a considerable overvoltage (at 25 ° C, if the current density is 200A/m2, the overvoltage of oxygen precipitation on copper is 0.605V). Therefore, the precipitation of oxygen cannot occur in the process of copper electrolytic refining. Only when the concentration of copper ions reaches a very high level or the anode in the electrolytic cell is severely passivated, so that the cell voltage rises above 1.7V, there may be oxygen on the anode. release. As for the discharge reaction of SO2-4 ionization, because its potential is corrected, it cannot be carried out in the process of copper electrolytic refining.
Metals such as Fe, Ni, Pb, As, Sb, which are more electronegative than Cu, dissolve into solution from the anode. The potential correction of precious metals does not dissolve and enters the anode slime. The precipitation potential of copper is more positive than that of hydrogen, and the overvoltage value of hydrogen precipitation on copper is very large (at 25°C and the current density is 100A/m2, the voltage is 0.584V), so only when the electrolyte near the cathode is in the electrolyte. The concentration of copper ions is extremely low, and hydrogen may be precipitated at the cathode only when severe concentration polarization occurs due to high current density.
The direct electrolytic refining process of scrap copper is a process in which copper is dissolved in the form of divalent on the anode under the action of direct current, and the divalent copper is precipitated in the form of metal on the cathode. During the electrolysis process, stable operating conditions should be maintained to obtain high-grade electrolytic copper.
The electrolytic refining of scrap copper uses frame anode, permanent stainless steel cathode plate or thin copper sheet (starter sheet) produced by electrolysis as cathode, and an aqueous solution of copper sulfate and sulfuric acid as electrolyte. Under the action of direct current, the anode copper is electrochemically dissolved, pure copper is deposited on the cathode, and impurities enter the anode slime and electrolyte, thus realizing the separation of copper and impurities.
Recommend products
CONTACT US:
If you have any requirement or suggestion, please fill in the form and send to us, thanks!E-mail:sunymachine@gmail.com | Whatsapp:+8613674945231








